Demystifying Dehumidification and Drying Part 1: Why Dry the Drying Air?
 As high summer begins to bring its heat and humidity in the Northern Hemisphere, many spray drying facilities have moved from their “winter rates” into their “summer rates” of production. Marked by reduced product throughput, increased fouling, and plain old misery for many facilities, summertime humidity can wreak havoc on spray drying performance. Every year, producers start to consider the potential of dehumidification for their spray drying facilities, but dehumidification for spray drying is a bit of a mystery for many production plants. This article is the first of a series in which we will try to demystify dehumidification – the physics behind it, the different methods of dehumidification, and its impacts on the drying plant. Let us dive in.
As high summer begins to bring its heat and humidity in the Northern Hemisphere, many spray drying facilities have moved from their “winter rates” into their “summer rates” of production. Marked by reduced product throughput, increased fouling, and plain old misery for many facilities, summertime humidity can wreak havoc on spray drying performance. Every year, producers start to consider the potential of dehumidification for their spray drying facilities, but dehumidification for spray drying is a bit of a mystery for many production plants. This article is the first of a series in which we will try to demystify dehumidification – the physics behind it, the different methods of dehumidification, and its impacts on the drying plant. Let us dive in.
Spray drying is at its very core exploiting the psychrometric properties of air and the relationship between humidity, temperature, and heat or enthalpy. We take air, heat it up, evaporate water from a liquid feedstock, and make a dryer product if everything goes right. This process is routinely mapped out on a psychrometric chart or Mollier diagram (which is just a psychrometric chart mirrored and turned 90°) to get a sense of what is happening.
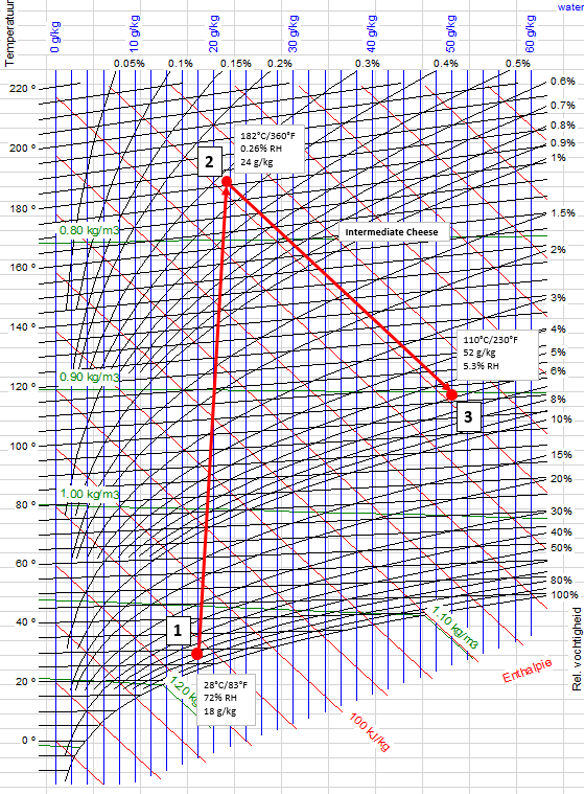
Figure 1. Mollier Curve for Spray Drying Plant
In Figure 1 above, we have a cheese powder that we are drying somewhere in the northern Midwest of the United States. This curve describes our drying process. We are drying this powder in the middle of the summer, during the hottest and humid part of the year. We draw in our ambient air [1] and we heat it up with a direct-fired burner [2], adding water to the drying air, to our desired drying temperature and outlet humidity [3]. We then introduce our liquid cheese and evaporate the water out of it, cooling the drying air as we do to our desired outlet temperature and relative humidity, careful not to go too far. We do not want to foul our dryer after all.
Based on this dryer design we are evaporating 28[1] grams of water per kilogram of drying air that we introduce into the dryer. Assuming we want to achieve an evaporation rate on the spray dryer of 2,000[2] kg/h we now know that we will need about 71,500 kg per hour of dry air. We certainly would want to have full capacity for the whole year, so we better make sure we can meet it in the summer. 71,500 kg/h it is. But what happens in the wintertime?
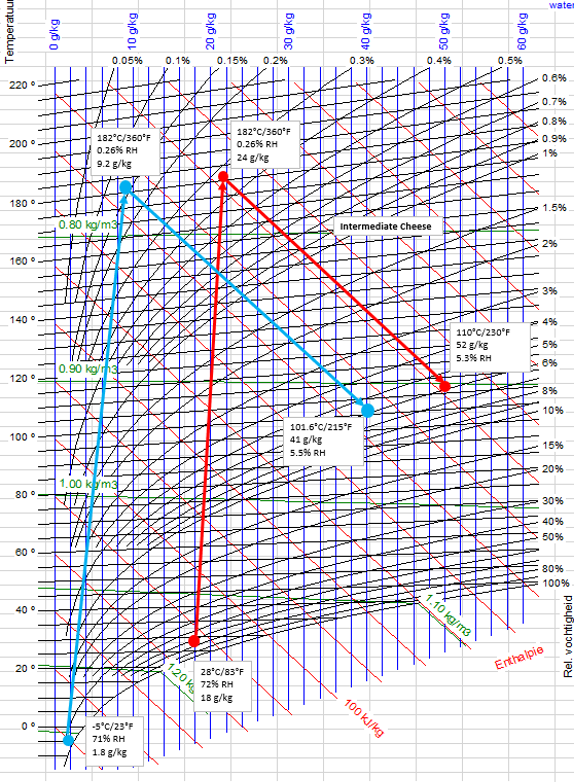
Figure 2. Mollier Curve for Spray Drying Plant Summer & Winter
Let’s map out our wintertime conditions for the dryer the same way we did with our summertime conditions, starting with the ambient air conditions, heating the air, and drying to roughly the same relative humidity as during the summer (we still don’t want to foul the dryer). We find that our outlet temperature has changed compared to what it is during the summertime. We will get into this in a later section, but for now, it is enough to know that this is due to the physics of the air.
The main take away here is that on a per unit mass basis, our evaporation in our spray dryer is almost 32 grams of water per kilogram of dry air. If we were to design our dryer based on these conditions, we would need 62,900 kg/h of dry air. But we know we cannot do this because during the summertime we have already determined that we need 71,500 kg/h of air. We surely cannot cut almost 10,000 kg/h of air from the design. We would come far too short on capacity. So, our spray dryer airflow is set to 71,500 kg/h.
With this airflow set for the design of our spray dryer, we will be able to achieve 12% more evaporation during the winter compared to the summertime design case, even with the colder inlet temperature! That is fantastic!
Eventually, however, this bonus of production in the wintertime will begin to feel like a lack of production capacity in the summertime. In our never-ending quest to maximize our asset utilization, what was the requested design point of the dryer becomes the handicap, all because of the requirements of design to meet capacity during the humid conditions of the summertime. But what if we could get rid of this extra moisture in the air during the summertime? We could utilize more of the maximum potential of the asset by shifting summertime ambient humidity into evaporative capacity. This is where dehumidification comes in.
While stabilization of annual production is generally the goal of dehumidifying, we could potentially realize our wintertime production rates year-round. For our plant here in the Midwest, this could mean an increase of 58 kg per hour during the worst part of the summer. Assuming this worst-case summertime increase for the entire month of July, at 65% availability of the asset (downtime for cleaning, maintenance, etc.), we are looking at an additional 28,000 kg of product produced in that single month alone. Assuming just half of this increase per hour as the average for our humid months of May to October, this is an additional 69 metric tons of product per year from our dryer. On top of this, the dryer will run at very similar conditions year-round which allows your staff to get used to and optimize processes even further.
Dehumidification is a potent option to stabilize production and increase the annual production capacity of existing spray drying – or any direct drying asset in which heated air is used to dry a feedstock. If considered from the beginning of a new project, dehumidification has the potential to reduce the excess capacity built into equipment, potentially reducing capital cost from the outset and helping to guarantee annual production rates. Please stay tuned for the next part of this series in which we dive into the details behind the physics of dehumidification.
Contact us for more information if you would like to know if dehumidification can work for your process, or for any other drying, evaporation, and thermal heat treatment questions.
[1] Divide grams by 1,000 and multiply by 7,000 to get to grains. In this case, 28 grams of water per kilogram of dry air is equal to 196 grains of water per pound of dry air (gr/lb.).
[2] Multiply kilograms by approx. 2.205 to get to pounds, in this case, 2,000 kilograms is equal to 4,410 pounds.
Read Part 2 of this series here.
